Spectroscopy of Ionic Molecules
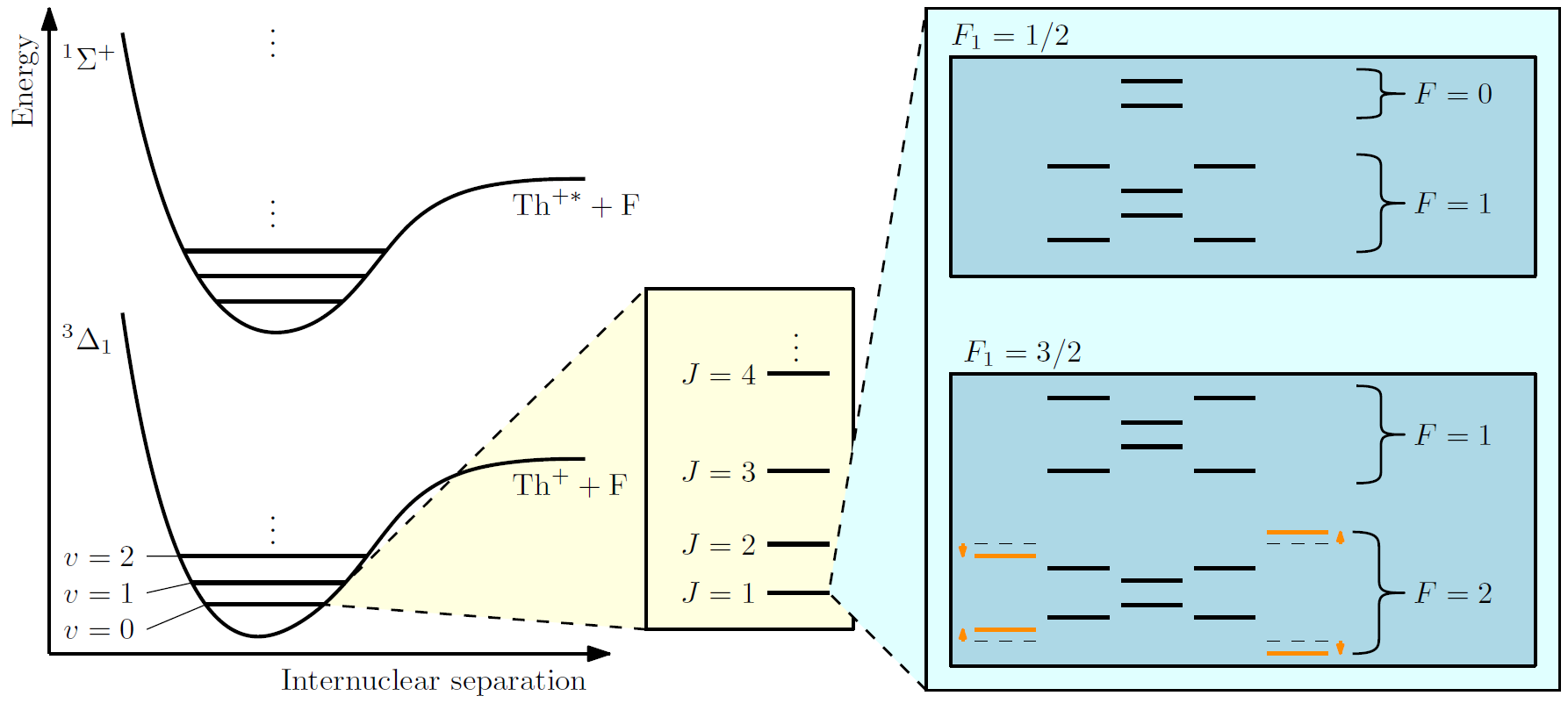 To measure something as small as the Schiff moment in a molecule, we must be able to (i) control it at the level of a single quantum level, and (ii) understand how the molecule responds to external fields. The former minimizes crosstalk from other quantum levels, which could wash away the small new-physics signal that we are interested in, and the latter allows us to exert a suitable level of control over our molecule to accomplish the former.
Both require us to perform spectroscopy of our molecule: shining lasers at our molecule and deciphering its internal structure through its response to the laser excitations.
Through the spectroscopy work, we can draw energy level diagrams like that shown on the right, with which we can make statements on how to perform quantum control of the molecule.
To measure something as small as the Schiff moment in a molecule, we must be able to (i) control it at the level of a single quantum level, and (ii) understand how the molecule responds to external fields. The former minimizes crosstalk from other quantum levels, which could wash away the small new-physics signal that we are interested in, and the latter allows us to exert a suitable level of control over our molecule to accomplish the former.
Both require us to perform spectroscopy of our molecule: shining lasers at our molecule and deciphering its internal structure through its response to the laser excitations.
Through the spectroscopy work, we can draw energy level diagrams like that shown on the right, with which we can make statements on how to perform quantum control of the molecule.
The effect of new physics can also be seen in the figure: new physics shifts the energy of the quantum levels of the molecule (example indicated by the orange lines). We aim to measure the minute shifts introduced by new physics.
Spectroscopy techniques of molecules have been very well established, but the steps are by no means trivial. One can easily spend a decade to perform the necessary spectroscopy required from scratch to understand the molecule well enough for a new physics measurement. At RadMol, we latch onto the great work of the JILA eEDM Gen. III group using singly charged thorium-232 monofluoride (232ThF+) in our investigation of singly charged thorium-227 monofluoride (227ThF+). These isotopologues (232ThF+ and 227ThF+) are chemically similar, hence their internal structures are largely similar (e.g., electronic, vibrational, and rotational degrees of freedom, for the experts). This means that most of the spectroscopy required is already done for 227ThF+. What we need to do is to walk the last mile by looking at the hyperfine structure that thorium-227 introduces with its nonzero nuclear spin (absent in thorium-232).
Currently we are on our way to perform spectroscopy on atomic thorium-227. After which, we will embark laser spectroscopy of 227ThF+ held in an ion trap. Through these experiments, we will also learn the necessary skills to perform spectroscopy on more complicated molecules, like triply charged protactinium-229 monofluoride.